NHRI Communications

健康知識
2013年中國大陸流感H7N9疫情及疫苗研發
Outbreaks of novel influenza H7N9 in China, 2013 and perspectives on vaccine development
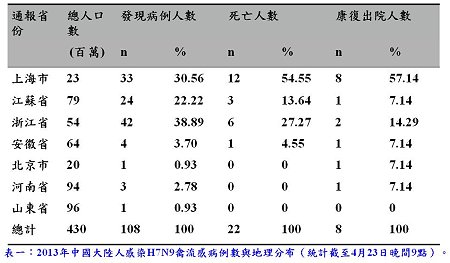 中國衛生單位於今年2月到3月間,在上海與安徽連續發現3例住院病例感染不明病原,且呈現嚴重下呼吸道症狀並死亡。經逆轉錄聚合酶鏈式反應(RT-PCR)確診後證明為H7N9禽流感感染。其中包括上海市一名87歲李姓老翁,其為首發病例,近期內無接觸禽鳥紀錄,其55歲及67歲的兒子皆呈現嚴重呼吸道症狀,父親與55歲兒子死亡,而另一名則已康復出院,經檢驗兩名兒子皆呈H7N9陰性,排除人傳人可能。另外兩名感染H7N9死亡的為上海市一名27歲從事家禽屠宰的男子以及安徽一名35歲家庭主婦,患病前曾造訪活禽市場。疫情在一個月內持續攀升,截至4月23日晚間9點,已造成108名人感染,22名死亡病例,分布遍及上海、江蘇、浙江、安徽、北京,河南與山東省 [1~5](表一)。多數 H7N9禽流感病例出現高燒、呼吸困難、咳嗽與嚴重肺炎症狀如急性呼吸窘迫症(fatal acute respiratory distress syndrome, ARDS)。然而,在北京一名4歲男童則被發現無症狀感染。H7N9 禽流感潛伏期為7天內,當前疾病致死率(case fatality rate, CFR)為19.5%,患者以男性為主,年齡高於 50歲者居多[1,6]。
中國衛生單位於今年2月到3月間,在上海與安徽連續發現3例住院病例感染不明病原,且呈現嚴重下呼吸道症狀並死亡。經逆轉錄聚合酶鏈式反應(RT-PCR)確診後證明為H7N9禽流感感染。其中包括上海市一名87歲李姓老翁,其為首發病例,近期內無接觸禽鳥紀錄,其55歲及67歲的兒子皆呈現嚴重呼吸道症狀,父親與55歲兒子死亡,而另一名則已康復出院,經檢驗兩名兒子皆呈H7N9陰性,排除人傳人可能。另外兩名感染H7N9死亡的為上海市一名27歲從事家禽屠宰的男子以及安徽一名35歲家庭主婦,患病前曾造訪活禽市場。疫情在一個月內持續攀升,截至4月23日晚間9點,已造成108名人感染,22名死亡病例,分布遍及上海、江蘇、浙江、安徽、北京,河南與山東省 [1~5](表一)。多數 H7N9禽流感病例出現高燒、呼吸困難、咳嗽與嚴重肺炎症狀如急性呼吸窘迫症(fatal acute respiratory distress syndrome, ARDS)。然而,在北京一名4歲男童則被發現無症狀感染。H7N9 禽流感潛伏期為7天內,當前疾病致死率(case fatality rate, CFR)為19.5%,患者以男性為主,年齡高於 50歲者居多[1,6]。病毒來源與基因分析
其實H7禽流感(H7N1、H7N2、H7N3、H7N7)感染人的案例,已於1996至2012年間發生於荷蘭、意大利、加拿大、美國、墨西哥與英國,卻未曾出現於亞洲。此次中國的人感染 H7N9 禽流感為全亞洲首度,亦為全世界首度發現人感染H7N9亞型禽流感 [6,7]。
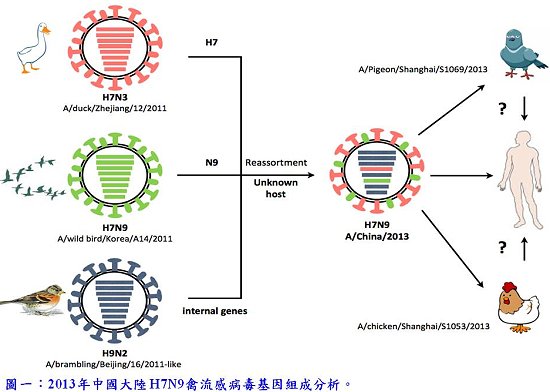
中國研究團隊在3月於全球共享禽流感數據行動聯盟(Global Initiative on Sharing Avian Influenza Data, GISAID)公布5個人感染H7N9禽流感全基因序列,兩個來自上海,其他來自安徽與浙江,包括 A/Shanghai/1/2013、A/Shanghai/2/2013、A/Anhui/1/2013、A/Zhejiang/DTID-ZJU01/2013 與A/Hangzhou/1/2013。另外亦已發表3株分別分離自鴿子、雞以及環境的H7N9禽流感基因序列。經序列分析結果顯示,此H7N9禽流感病毒乃重組自3個歐亞型禽流感病毒。其血球凝集素(Hemagglutinin, HA)亞型H7的基因片段源自於浙江的鴨子禽流感病毒(H7N3),而神經氨酸酶(Neuraminidase, NA)亞型N9則來自韓國的野鳥禽流感病毒(H7N9);另外6段基因則源自長江以北的燕雀或雞隻禽流感(H9N2)[6]。這3個來源的病毒在未知的宿主中進行基因交換,而後感染人、雞與鴿子 (圖一)。特別值得注意的是,HA基因分析結果顯示,此H7N9禽流感於禽類為低致病性。不像高致病性 H5N1禽流感,我們可由大規模禽類死亡來預估可能的人感染疫情,而此H7N9禽流感可能由無症狀的禽類帶毒,悄悄地散布,進而對人類健康造成嚴重危害[8]。 然而,是否還有其他動物易遭受影響,如何感染人的傳播途徑皆尚未明朗,因此需增加對人類及動物的流感疾病監測,才能釐清傳染源,進而有效地防治H7N9的流行。
人傳人的風險?
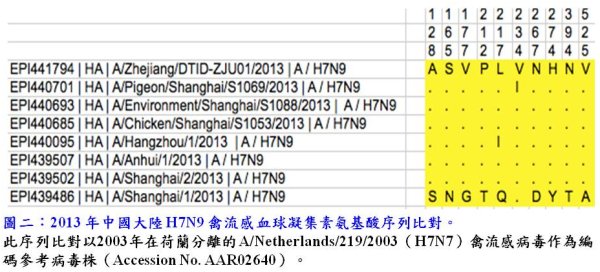
雖然目前的流行病學調查無法證實2013年在中國流行的H7N9禽流感已獲得人傳人的能力,但氨基酸序列分析結果顯示,部分氨基酸的位點上已從類禽流感轉變成類人流感病毒。以HA來說,其氨基酸序列226號位置若由穀氨酰胺(Glutamine, Q)變成亮氨酸(Leucine, L; Q226L, H3 numbering),則病毒對宿主細胞表面受體的結合,則會由結合α-2,3唾液酸多醣受體(α-2, 3 sialic acid, SA)改變為傾向結合α-2, 6 SA。而α-2, 3 SA 受體多處於禽鳥與人類下呼吸道,α-2, 6 SA受體則多處於哺乳動物細胞表面與人類上呼吸道上皮細胞。根據HA基因分析,目前中國的H7N9病毒有2群(圖二),此2群病毒的HA基因大約有10個氨基酸差異。除了A/Shanghai/1/2013,其他3株自人分離的 H7N9禽流感病毒皆已具有Q226L變異,此變異可能導致H7N9禽流感病毒較易感染人類與哺乳動物,並獲得飛沫傳染的能力,大大增加人傳人的危險性。除了Q226L(Q217L依據H7編碼),H7N9病毒的HA上發現其他位點變異包括S138A、T160A與G186V,皆增加了病毒結合人受體的能力[6,9,10]。
除了HA外,H7N9病毒PB2蛋白上發現了E627K 變異,過去文獻證明此變異對增加病毒對小鼠(哺乳動物)的毒性,並使病毒更適合存活於類近人體體溫的溫度,而容易在哺乳動物宿主複製。與人流感及一般禽流感病毒相比,於H7N9病毒的NA上69-73號氨基酸發現短少,而此現象已證明會增加對小鼠的毒性。除此之外,在H7N9病毒易發現其M1蛋白上的N30D、T215A,以及 NS1蛋白上的P42S 位點突變,皆會增加病毒對小鼠的毒性。
另外,某些氨基酸位點改變亦會增加病毒的抗藥性。H7N9病毒的NA蛋白上已發現R294K位點突變,此突變會增加病毒對oseltamivir(克流感,Tamiflu)與 zanamivir(瑞樂沙,Relenza)的抗性;而在其M2蛋白上發現的S31N會增加病毒對金剛胺(amantadine)與金剛乙胺(rimantadine)的抗性[9,10]。截至目前,已有多起家庭群聚感染H7N9案例,各方專家正努力追查並釐清是否有人傳人之實例。
H7N9 禽流感人用疫苗開發
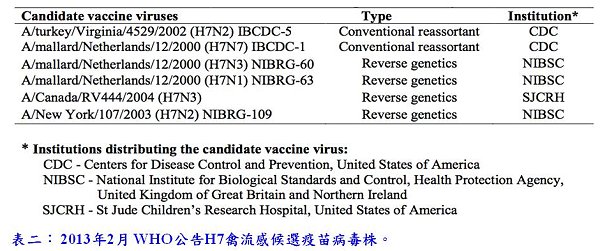
鑒於H7禽流感於1996至2012年在各地引起零星的禽傳人病例,世界衛生組織(WHO)曾製備出6株H7禽流感疫苗候選病毒株[11](表二)。法國賽諾菲公司曾利用美國疾管局製備H7N7疫苗種株,以雞胚胎平台生產裂解疫苗(季節性流感疫苗配方)進行臨床試驗,結果發現H7N7裂解疫苗致免疫力很差,即使每劑90微克,接種兩劑之後,只有5%產生免疫反應[12];此外,法國賽諾菲公司也曾利用細胞培養技術生產H7N1裂解疫苗進行臨床試驗,結果也顯示未含佐劑的疫苗,免疫反應很差,不過接種2劑(每劑HA抗原12或24微克)含佐劑(氫氧化鋁)的疫苗,抗體反應分別為43%和46%[13];美國MedImmune公司也曾進行H7N3活性減毒疫苗的臨床試驗,也顯示H7N3疫苗的免疫效果不佳。綜合來看,流感H7疫苗似乎與H5疫苗比較相似,需要全病毒疫苗搭配佐劑才能有較佳免疫反應。本單位序列分析結果發現,H7N9禽流感HA序列與這些疫苗株的HA基因差異大於15個氨基酸以上,因此它們之間交叉保護力可能很低。中國大陸已於4月11日左右提供1株野生H7N9病毒株(A/Anhui/1/2013)給WHO的6個參考實驗室製備疫苗種株,根據HA基因分析,目前中國流行的H7N9病毒包含2群,因其抗體交叉反應不詳,最好2群病毒皆應製備疫苗種株。此外,仍然不知H7N9疫苗的最佳配方,因此,目前當務之急,是儘速製備出疫苗種株,利用不同技術平台生產不同配方的疫苗,進行臨床試驗,如此才能決定疫苗生產的方向,以有效防治H7N9今年冬天可能造成的流行與危害。
參考文獻
- 中國衛生部衛生應急辦公室(突發公共衛生事件應急指揮中心 -人感染 H7N9禽流感防控工作. moh.gov.cn.)(accessed 17 Apr. 2013).
- FluTrackers 2013 Test Positive Human Case List for Confirmed and Highly Suspected/Probable H7N9 Type Influenza - FluTrackers. flutrackers.com. (accessed 17 Apr. 2013).
- Influenza A virus subtype H7N9 - Wikipedia, the free encyclopedia. en.wikipedia.org. (accessed 17 Apr. 2013).
- 截止4月17日21時全國H7N9禽流感病例分布圖 dituhui.com.
- Avian influenza A virus H7N9- Virology Down Under. uq.edu.au.
- Gao R, Cao B, Hu Y, et al. Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. The New England Journal Medicine 2013;130411140151005.
- WHO | Frequently Asked Questions on human infection with influenza A(H7N9) virus, China. WHO
- Centers for Disease Control & Prevention. H7N9_summary_data_05APR2013. 2013; 1–12.
- Uyeki TM, Cox NJ. Global Concerns Regarding Novel Influenza A (H7N9) Virus Infections. The New England Journal Medicine2013;1–3.
- Kageyama T, Fujisaki S, Takashita E, et al. Genetic analysis of novel Avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance;18.
- WHO | Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. WHO
- Couch R. B., Patel S. M., Wade-Bowers C. L, et. al. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS ONE2012;7(12):1-6.
- Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009 Mar 18;27(13):1889-97. PubMed PMID: 19368768.
- Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine. 2009 Jun 8;27(28):3744-53. PubMed PMID: 19464558. Pubmed Central PMCID: 2771405.
《文/圖:感染症與疫苗研究所黃培妤研究助理、李敏西副研究員、王雅芳博士、蘇益仁所長》